What is CAR-T and How Does It Work
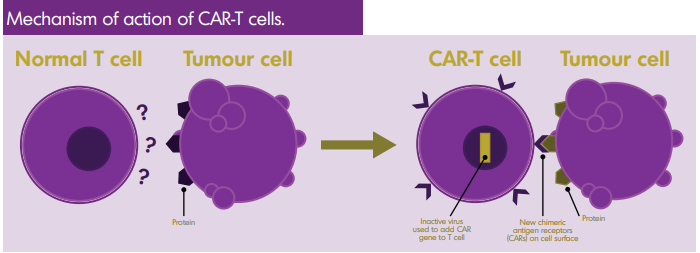
Chimeric Antigen Receptor T cell therapy, or CAR-T therapy, is a type of immunotherapy that modifies a patient’s T-cells to better detect and destroy lymphoma. A part of the body’s immune system, T cells are a type of white blood cell that attacks virus-infected cells, foreign cells and cancer cells. CAR-T therapy enhances the effectiveness of T cells.
CAR-T therapy uses genetic engineering to alter a patient’s T cells. A virus is used to penetrate the T cell and genetic material is inserted. This produces transmembrane proteins on the T cell’s surface that recognize specific proteins in the tumour. The target for CAR-T therapy can be chosen when the cells are being engineered. Currently, CAR-T therapy against CD19 (a marker seen in most B cell lymphomas) has become a new, standard treatment.
With current CAR-T therapies, two signals are created through CAR-T cells: one activates the T cell when it binds to the tumour’s protein; the second stimulates cellular replication, which ensures a significant supply of chimeric T cells in the patient.
Who is CAR-T For?
CAR-T currently treats some aggressive subtypes of lymphoma. In addition to subtype and stage, physicians will also assess the patient’s overall health, fitness and circumstance for this treatment. This may include the patient’s comorbidities, the associated ability to withstand the vigorous treatment, and their willingness and ability to travel for treatment. There are specific eligibility criteria for each CAR-T therapy, based on what was learned in clinical trials and regulatory approvals.
Generally speaking, the following patients are eligible for CAR-T therapy:
- Were diagnosed with a subtype of lymphoma for which the CAR-T therapy is approved
- Meet regulatory criteria (e.g. relapsed twice and have been previously treated)
- Have tumours that express the specific antigen the therapy targets
- Satisfactory organ function and performance status
- No active infections
- No significant cardiac, neurological or immune dysfunction
Physicians will discuss patient’s eligibility and will send relevant medical records to the specialty cancer
centre that performs the CAR-T therapy to be evaluated for treatment.
Clinical Effectiveness
Understanding patient outcomes after treatment with CAR-T therapy is important because while the initial results for some patients are positive, clinical effectiveness varies considerably amongst patients. The ideal patient candidate is still under trial. Some patients achieve long-term durable remission, but CAR-T therapy is not currently considered a cure as more data is needed to see if patients who achieve a full remission stay disease-free long-term. Currently available 4-year survival data for DLBCL patients shows promising results thus far.
The outcomes measured in clinical trials include:
Complete response rate (CR)
- The percentage of patients who respond to treatment with the disappearance of all signs of cancer. Even if patients do not achieve CR, they could experience a partial response (PR). Overall response rate (ORR) is the sum of CR and PR.
Median overall survival (mOS)
- The median length of time from the date of diagnosis or the start of treatment that patients are still alive.
Progression-free survival (PFS)
- The length of time during and after treatment that a patient lives with disease but it does not progress or get worse.
Side Effects
There are several short- and long-term side effects related to CAR-T therapy. Patients are advised to stay near their treatment centre for a few weeks. Most short-term side effects can be managed with supportive therapies. However other side effects can be more serious and potentially life-threatening and may require treatment in a hospital intensive care unit.
Cytokine Release Syndrome
As CAR-T cells are released and multiply in the patient’s body, their immune system is highly activated and releases a massive number of inflammatory cytokines into the blood, causing cytokine release syndrome (CSR). This can happen within the first week after infusion or later in some cases.
The duration and intensity of CSR can vary. Some patients only experiencing mild flu-like symptoms while others have high fevers, hypoxia (oxygen deficiency), low blood pressure and multi-organ toxicity.
Neurotoxicity (now termed IEC-associated neurotoxicity syndrome or ICANS)
IEC refers to Immune Effector Cells. The CAR-T cells can have an effect on the patient’s brain, which can cause confusion, agitation and a lack of awareness. Patients may experience headaches, difficulties with written or spoken language, anxiety and occasional seizures.
Neurotoxicity can occur with or without cytokine release syndrome. If a patient also experiences CRS, neurologic symptoms more commonly occur after CRS. It is reversible in most cases, but in rare cases, cerebral oedema associated with neurotoxicity may develop, which is potentially fatal.
Macrophage-Activation Syndrome
The severe inflammation of the immune system, which can cause multi-organ failure.
Febrile Neutropenia
An abnormal decrease in the number of certain white blood cells in the blood coupled with fever.
B Cell Aplasia
Low number of B cells or no B cells.
Anaemia
A condition in which the number of red blood cells is below normal.
Thrombocytopenia
A decrease in the number of platelets in the blood.
Hypogammaglobulinemia
A reduction in all types of gamma globulins, including antibodies that help fight infection.
Severe side effects are often the result of the high activity of the immune system brought on by the CAR-T therapy. Immunosuppressive drugs and corticosteroids are used in symptom management, with the goal of curtailing the side effects without removing the CAR-T cells completely.
It is important patients and caregivers can identify symptoms quickly so they can be treated appropriately, especially since the symptoms of some side effects (like neurotoxicity) are the same as those of other medical conditions which are treated very differently.
How to Access CAR-T Therapy and Existing Challenges
CAR-T therapy is still considered a new and innovative therapy for the treatment of lymphoma. It was initially approved by Health Canada in 2018 for relapsed/refractory Diffuse Large B-Cell Lymphoma patients, and more recently in 2021 for Mantle Cell Lymphoma, however it is still not locally accessible for all patients across Canada. This remains a major challenge with this therapy, as it is only currently locally accessible for patients with DLBCL in Ontario, Quebec, Alberta and Nova Scotia.
However, this does not mean that patients from other provinces cannot access this treatment. Inter-provincial agreements have been developed so that patients in a province without access can travel to a province with this therapy.
Challenges that have contributed to volume and local access include:
Availability
CAR-T therapy can only be administered in specialty cancer centres who have been certified to provide the therapy, which limits its availability. Patients who are eligible for the therapy may have to travel from and stay away from home for several weeks – for blood sampling, during infusion and through the observation period. This can be both disruptive and expensive for patients and carers.
Cost of treatment
There is a significant financial cost associated with CAR-T therapy, both for the therapy and associated medical services. While some manufacturers have created outcome-based pricing arrangements, with the company only receiving payment if the patient responds to the treatment, the cost of CAR-T therapy is significant to payers – public programs, insurance programs and individual patients alike.
As a result of some of these challenges, there are some provinces that are developing made-in-Canada CAR-T products, however many of these are still in clinical trial testing.
Lymphoma Canada is working hard to advocate for local access to CAR-T therapy for eligible patients in provinces where there are barriers and delays. Having local access is important for patients as:
- the patient can remain close to their family/friend support group and network
- the patient will have proper follow-up care with their treating physician
- there are more limited financial impacts and support available
- there is a reduced risk of COVID-19 exposure due to travel
It is important to also note that there can be challenges with the therapy and its administration, and research is ongoing to better understand CAR-T therapy and its long-term impact for patients.
Varied results
While some patients respond to CAR-T therapy very well and their disease goes into remission, there are still others that don’t and it’s unknown why. Who is the ideal candidate for CAR-T therapy? Are there genetic or clinical factors make one patient a better candidate than another? Do prior therapies impact results? Are there predictors that can help gauge a patient’s response?
Wait time to treatment
As current CAR-T therapy is customized to individual patients, there is a time-lapse between when patients are accepted to receive CAR-T until their manufactured T cells are returned for infusion. As patients currently eligible for this treatment are often quite ill, it can be challenging
Research
Research and testing for CAR-T therapy is ongoing with hundreds of clinical trials open around the world to test CAR-T therapy in different lymphoma subtypes and lines of therapy (i.e. 2nd line treatment, 3rd line treatment, etc.). With CAR-T therapy approved by Health Canada for Diffuse Large B-Cell Therapy and Mantle Cell Lymphoma, CAR-T testing is currently ongoing for major lymphoma subtypes including Follicular Lymphoma, Chronic Lymphocytic Leukemia, Burkitt Lymphoma, Marginal Zone Lymphoma, Lymphoplasmacytic Lymphoma, Waldenstrom Macroglobulinemia, transformed lymphomas, etc.
Stay up to date with clinical trial testing for CAR-T therapy through clinicaltrial.gov or canadiancancertrials.ca.
Important research to highlight on the advancement of CAR-T therapy in lymphoma:
- Hirayama, A. et al. (2019). High rate of durable complete remission in follicular lymphoma after CD19 CAR-T cell immunotherapy. Blood 134(7):636-640. Access the research article here.
- Turtle, C.J. et al. (2017). Durable Molecular Remissions in Chronic Lymphocytic Leukemia Treated With CD19-Specific Chimeric Antigen Receptor-Modified T Cells After Failure of Ibrutinib. J Clin Oncol. 35(26):3010-3020. Access the research article here.
- Zhou, X. et al. (2021). CAR19/22 T cell therapy in adult refractory Burkitt’s lymphoma. Cancer Immunol Immunother, 70(8):2379-2384. Access research abstract here.